The medical grants ecosystem: people, programs, and products

Medical grantmaking is a complex web of programs and people — where each piece plays a vital role.
Expanded access programs deliver life-saving treatments, research grants push scientific breakthroughs, and medical education drives better health outcomes. The people behind these efforts range from doctors and patients seeking treatments to investigators and researchers securing funding. At the heart of it all is the platform where grant administrators keep things moving: reviewing applications, managing payments, ensuring compliance, tracking impact, and more.
We call this network of people, programs, and tools the “ecosystem of care,” with every piece working toward one goal: saving more lives.
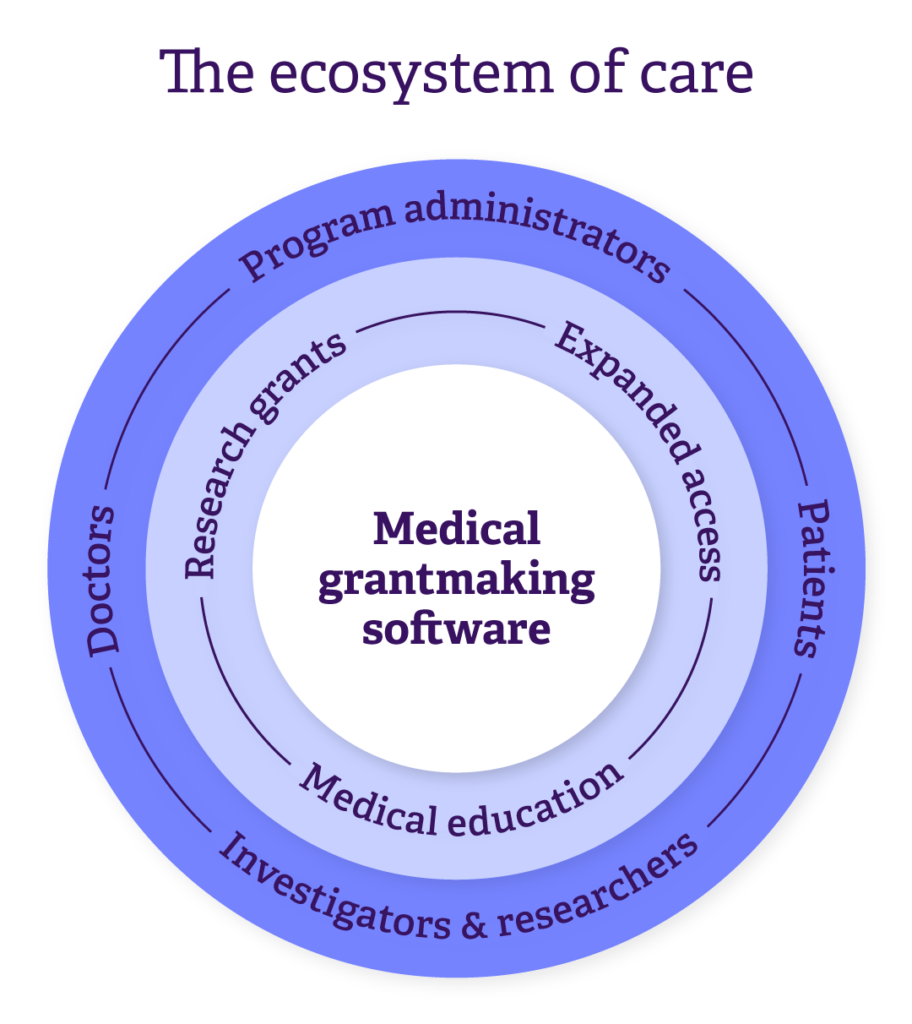
In this blog, we’ll explore how our grantmaking software is designed with this ecosystem in mind, ensuring it meets the unique needs of medical grant programs and the stakeholders driving them forward.
Expanded access: unlock faster submissions and approvals
Accelerate submission, review, and approval processes for faster treatment access.
For expanded access programs, each product or treatment typically has its own approver, making manual routing tedious and time- consuming for admins. These programs are also governed by global regulations, requiring careful oversight and strict compliance.
Beyond that, requests for access often require a quick turnaround. The decision whether to treat a patient facing the final stages of an illness must be made in a matter of days, if not hours. As a result, speed must be carefully balanced against oversight.
That’s where Bonterra’s Expanded Access software comes in. It makes submitting and approving requests faster, smoother, and fully compliant. Here’s how:
- Streamlined submissions: Healthcare providers can submit treatment requests with test results and supporting documentation in a format optimized for quick review.
- Automatic routing: Approvers are automatically sent applications and relevant documents based on location or medication type, slashing manual tasks.
- Increased visibility: Admins can track every step of the process — from form submission to drug shipment — for compliance and audit-readiness.
Research grants: improve communication and transparency
Automate communication between researchers and funders for faster payments.
Research grants are typically paid out in stages, with milestone payments released throughout the study rather than all at once. That means funders need an easy way to stay connected with investigators, track progress, and access updates in real- time. Without it, finding critical data and forecasting payments becomes more difficult than it should be, wasting time and delaying funding.
Bonterra’s Research Grants solution makes staying connected easy. Here’s how:
- Foster stronger partnerships: Strengthen trust and alignment with built-in, trackable communication tools that keep researchers and funders on the same page.
- Reduce risk: Automatically track communications and milestones to ensure compliance with global regulations.
- Increase transparency: Use tailored dashboards and data visualizations to easily share milestone updates and insights with key stakeholders.
Medical education: track the impact of your initiatives
Show funders how medical education programs lead to better healthcare outcomes.
Funders want to support medical education programs to improve disease management, patient safety, population health, and more. But in order to secure funding, program admins need to show their initiatives are effective and worth the investment.
Without a tool that can automatically track progress toward goals and help extract key outcomes, measuring success can become a tedious, time-intensive process. Here’s how Bonterra’s Medical Education software can help simplify reporting:
- Ensure compliance: Easily track detailed information on budget, attendance, progress, and outcomes to ensure your medical education initiatives comply with regulations and standard operating procedures (SOPs).
- Measure outcomes: Use automated reports and data visualizations to quickly compare actual vs. expected results, empowering you to make data-driven decisions and show your program’s impact.
Purpose-built software for users across the ecosystem of care
Bonnterra’s medical grants software is built with the needs of every person and program in mind. That means faster processing for expanded access requests, real-time visibility into research grant milestones, and the tools to grow funding for medical education programs. Partner with us for medical grants management to unlock these benefits and more.
Ready to see it in action? Schedule a demo and discover why our life sciences software helps companies of all sizes— from early-stage to some of the largest in the industry — trust Bonterra for medical grants management.
Corporate Social ResponsibilityCorporations,Life sciencesMedical,affairs
Work with Bonterra



